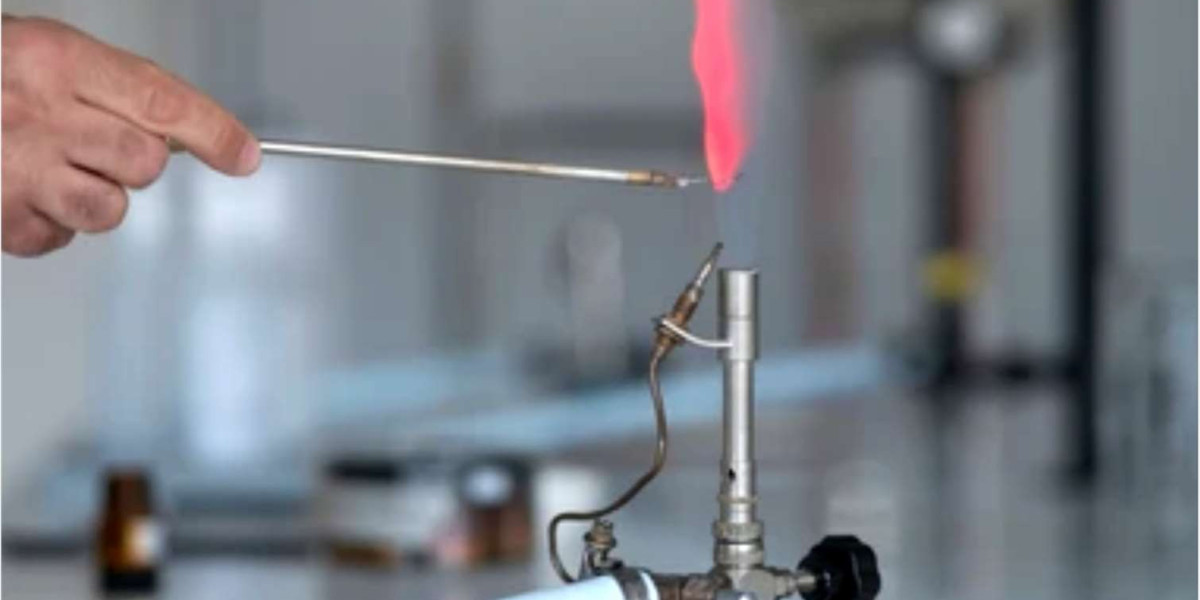
The plastic material burn test serves as a fundamental process for recognizing different plastics through their distinct combustion properties. Multiple industries require knowledge of plastic material burn characteristics for effective recycling processes and material selection while assessing environmental impacts. The simplified guide from Alfa Chemistry enables users to identify common plastic materials by employing a flame source like a lighter, torch, or bottled gas burner.
What is a plastic material burn test?
The burn test requires placing a flame against a plastic sample to monitor its reaction. The straightforward test enables clear identification between thermoplastic and thermoset materials. This burn test provides initial information about the material but fails to deliver a conclusive identification by itself. Advanced methods, including spectral analysis, are necessary to identify complex polymers accurately.
The burn test delivers vital data, including:
- Flame Color: The resulting flame color reveals information when a material undergoes combustion. The material's chemical composition determines the flame color during combustion. A blue flame usually suggests thorough combustion with fewer impurities, but yellow or orange flames demonstrate the presence of carbon or other contaminants in the material.
- Soot Presence: Whether the burning plastic produces soot. The generation of soot during combustion demonstrates incomplete burning, which occurs frequently in plastics containing high carbon content and various additives.
- Odor: The smell produced when the plastic burns. The aroma emitted from burning plastic serves as a diagnostic tool to identify the specific type of polymer used.
- Material Drips: Whether the plastic drips during combustion. Thermoplastics drip during combustion because they become pliable when exposed to heat. Unlike thermoplastics, which drip when heated, thermosets maintain their form and do not drip when exposed to flame.
- Smoke Characteristics: The type and amount of smoke emitted.
How to perform a plastic material burn test?
These steps should guide you through performing the initial burn test for identification.
1. Thermoplastic vs Thermoset Identification
Determining the material type as thermoplastic or thermoset comes before beginning the burn test. To perform this test, you need to use a heated metal or glass rod.
- Bring the rod to a glowing state at approximately 500°F/260°C before proceeding.
- Press the heated rod against the sample.
- Materials that soften when exposed to heat are generally classified as thermoplastics. Materials that remain unchanged after exposure to heat are typically thermoset.
2. Flame Exposure
Place the sample near the edge of flames from sources including cigarette lighters, gas burners, or torches. Hold the material in the flame for a maximum of ten seconds if it fails to ignite right away. Carefully observe:
- Color of the Flame: Analyzing the color of the flame during the test can reveal information about what the material is made of.
- Nature of Smoke: The density and color of the smoke produced during burning serve as indicators for identifying the specific type of plastic.
- Presence of Soot: The development of soot during combustion demonstrates incomplete burning, which suggests the material remains partially unoxidized.
3. Odor Analysis
Once the flame has been extinguished, proceed to carefully smell the resulting fumes. The burning process of each plastic material produces a unique smell. PVC emits a sharp acrid smell when burned, but burning polyethylene releases a sweet and gentle odor. Comparison references provide assistance in determining the sample's identity.
4. Dripping Test
Plastics may release dripping droplets during combustion, with thermoplastic materials being particularly prone to this behavior. The particular feature plays a key role when differentiating among the various plastic kinds.
How do common plastics react under burn test conditions?
The data from plastic burn tests plays a crucial role in choosing appropriate materials and improving recycling methods. This table provides a summary of burn characteristics found in common thermoplastic and thermoset materials.
| Materials | No Flame | Burns, but goes out when the source of fire is removed | Continues to burn when the source of fire is removed | Remarks | |||||
| Odor | Odor | Flame Color | Drips | Odor | Flame Color | Drips | Speed of Burning | ||
| Thermoplastics | |||||||||
| ABS | Acrid | Yellow, blue edges | No | Acrid | Yellow, blue edges | Yes | Slow | Black smoke with soot in air | |
| ABS/Polycarbonate | - | - | - | - | - | Yellow, blue edges | No | - | Black smoke |
| ABS/PVC | - | Acrid | Yellow, blue edges | No | - | - | - | - | Black smoke with soot in air |
| Acetals | - | - | - | - | Formaldehyde | Blue, no smoke | Yes | Slow | |
| Acetate | - | Vinegar | Yellow with sparks | No | Vinegar | Yellow | Yes | Slow | Flame may spark |
| Acetate Butyrate | - | - | - | - | Rancid butter | Blue, yellow tip | Yes | Slow | Flame may spark |
| Acrylics | - | - | - | - | Fruity | Blue, yellow tip | No (cast) Yes (molded) | Slow | Flame may spurt if rubber modified |
| Chlorinated Polyether | - | Green, yellow tip | No | - | - | - | - | Black smoke with soot in air | |
| CTFE | Faint odor of acetic acid | - | - | - | - | - | - | - | Deforms; no combustion, but drips |
| Ethyl Cellulose | - | - | - | - | Burnt sugar | Yellow, blue edges | Yes | Rapid | - |
| FEP | Faint odor of burnt hair | - | - | - | - | - | - | - | Deforms; no combustion, but drips |
| Modified Grade | - | Phenol | Yellow-orange | No | - | - | - | - | Flame spurts; difficult to ignite, soot in air |
| Nitrate | - | - | - | - | camphor | White | No | Rapid | - |
| Oxides (PPO) | - | Phenol | Yellow-orange | No | - | - | - | - | Flame spurts; very difficult to ignite |
| Phenoxies | - | Acrid | Yellowc | No | Acrid | Yellow | Yes | Slow | Black smoke with soot in air |
| Polycarbonates | - | Faint, sweet aromatic ester | Orange | Yes | - | - | - | - | Black smoke with soot in air |
| Polyethylenes | - | - | - | - | Paraffin | Blue, yellow tip | Yes | Slow | Floats in water |
| Polyimides | - | - | - | - | - | - | - | - | Chars; material very rigid |
| Polypropylenes | - | Acrid | Yellow | Yellow | Sweet | Blue, yellow tip | Yes | Slow | Floats in water; more difficult to scratch than polyethylene |
| Polystyrenes | - | - | - | - | Illuminating Gas | Yellow | Yes | Rapid | Dense black smoke with soot in air |
| Polysulfones | - | - | Orange | Orange | - | - | - | - | Black smoke |
| Polyurethanes | - | - | - | - | - | Yellow | No | Slow | Black smoke |
| Propionate | - | - | - | - | Burnt sugar | Blue, yellow tip | Yes | Rapid | - |
| PTFE | Faint odor of burnt hair | - | - | - | - | - | - | - | Deforms; does not drip |
| PVC/Acrylic | - | Fruity | Blue, yellow tip | No | - | - | - | - | |
| PVDF | acidic | - | - | - | - | - | - | - | Deforms |
| Rigid | - | Hydrochloric acid | Yellow with green spurts | No | - | - | - | - | Chars, melts |
| Type 6 | - | - | - | - | Burnt wool | Blue, yellow tip | Yes | Slow | - |
| Type 6/6 | - | Burnt wool or hair | Blue, yellow tip | Yes | - | - | - | Slow | More rigid than Type 6 nylon |
| Vinyls Flexible | - | Hydrochloric acid | Yellow with green spurts | No | - | - | - | - | Chars, melts |
| Thermosetting plastics | |||||||||
| Diallyl Phthalates | - | - | - | - | Phenolic | Yellow | No | Slow | Black smoke, cracks |
| Diglycol Carbonate | - | - | - | - | Acrid | Yellow | No | Slow | Black smoke with soot |
| Epoxies | - | - | - | - | Phenol | Black smoke | No | Slow | Black smoke with soot in air |
| Melamines | Formaldehyde and fish | - | - | - | - | - | - | - | |
| Phenolics | Formaldehyde and phenolc | Phenol and wood or paperd | Yellow | No | - | - | - | - | May crack |
| Polyesters | - | Hydrochloric acida | Yellow | No | - | Yellow, blue edges | No | Slow | Cracks and breaks |
| Silicones | - | - | - | - | - | - | - | - | Deforms |
| Ureas | Formaldehyde | - | - | - | - | - | - | - | - |
How accurate is the plastic burn test for identification?
The burn test offers a rapid preliminary plastic identification technique but is not completely reliable. The presence of additives like flame retardants modifies how materials behave when burned. Composite materials that include multiple plastics or fillers exhibit burn behaviors that blend together, making identification hard without detailed examination.
Alfa Chemistry advises using the burn test together with advanced techniques like infrared spectroscopy or pyrolysis-gas chromatography to achieve accurate material identification. These methods enable a detailed chemical analysis of plastic materials, which leads to accurate identification of complex and composite materials.